ADDITION POLYMERIZATION


- The individuals unit that polymerizes to form a polymers is known as a monomers. Eg Ethene
- The structure formed by the polymerization of the monomer is a polymers.
Polymers are materials made by linking up smaller repeating chemical units.
Some bend and stretch – rubber and polyester.
Some hard and tough – epoxies and glass.
ADDITION POLYMERS
- a) Formed by addition reaction.
- b) Require only one monomer generally an alkene
- c) Nothing is lost in the reaction.
eg Polyethene, polypropene
CONDENSATION POLYMERS
- a) Requires two monomers
- b) Requires two functional group
- c) Formed by condensation reaction.
- d) A small molecule of water is
- e) Example: Nylon a polyester
Synthetic Polymer
Edexcel IGCSE Chemistry Synthetic Polymer
FREE DOWNLOAD
Send download link to:
Edexcel IGCSE Chemistry Synthetic Polymer
FREE DOWNLOAD
Send download link to:
NATURAL POLYMERS
- a) They are found naturally
- b) All the complex biomolecules are polymers
| Monomer | Polymer |
| Glucose | Starch |
| Proteins | Amino Acid |
| Nucleotide | DNA |

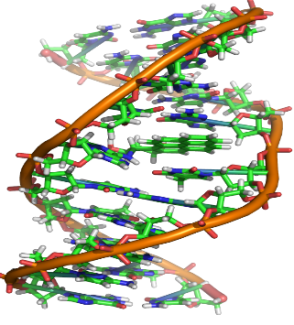
DNA
- a) DNA is polynucleotide
- b) Nucleotide = Phosphate + Sugar + Nitrogenous Bases
- c) There are four bases present in the DNA
- Adenine
- Thymine
- Guanine
- Cytosine

DISCLAIMER
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons

