CRUDE OIL
- It is a black thick liquid which takes millions of years to form.
- It is the mixture of hydrocarbon.
- Hydrocarbon are the compounds made up of carbon and hydrogen only.
- The components of the crude oil are important and the crude oil is separated by the process of fractional distillation.

HYDROCARBON PROPERTIES

FRACTIONAL DISTILLATION OF CRUDE OIL
- Separating the mixtures on the basis of boiling points.
- It is separated in fractionating column with different substances of similar boiling points

| LIQUIFIED GAS | FUEL |
| GASOLINE/PETROL | CAR FUEL |
| KEROSENE | AIRCRAFT FUEL |
| DIESEL OIL | FUEL IN DIESEL ENGINES |
| RESIDUE | MAKING ROADS |
L – Look
G – Great
K – Kid
D – Doing
R – Roll
CRACKING –Thermal decomposition of longer chain hydrocarbon into a shorter chain alkane and alkenes
Thermal Cracking Catalytic Cracking
It is done at a very high temperature It is done using a catalyst

CRUDE OIL
Edexcel IGCSE Chemistry Crude Oil
FREE DOWNLOAD
Send download link to:
Edexcel IGCSE Chemistry Crude Oil
Send download link to:
WHY CRACKING
- Shorter chain alkanes are more in demand as they are more efficient fuel which fractional distillation alone cannot meet.
- Alkenes are required for polymerization and synthesize other hydrocarbons which fractional distillation cannot meet.
ALKANES – Saturated Hydrocarbon
Carbon-carbon single bond made up of carbon and hydrogen
General Formulae CnH2n+2
Methane – CH4
Ethane – C2H6
Propane – C3H8
Butane – C4H10
Pentane – C5H12
Homologous Series – Members of the same family have similar functional group similar chemical properties and general formulae but different physical property and each members differs from successive by CH2.
COMBUSTION
COMPLETE
| INCOMPLETE
|
| FUEL IS COMPLETELY BURNED | FUEL IS PARTIALLY BURNED DUE TO LIMITED SUPPLY OF OXYGEN
|
| PRODUCES CARBON DIOXIDE AND WATER | PRODUCES CARBON MONOXIDE AND WATER
|
| IT IS NOT TOXIC | CARBON MONOXIDE IS TOXIC AS IT DECREASES. THE OXYGEN CARRYING CAPACITY OF RED BLOOD CELLS
|
PRODUCTS OF COMBUSTION
Carbon Dioxide Test

Limewater Test Carbon Dioxide will turn limewater milky
Water Test



FUNCTIONAL GROUPS
Groups of atoms that give special properties and reactions to the organic molecule
| Functional Groups | Examples | Formation | |
| ALKENES | = | Ethene, propene, butene, pentene | Cracking of crude oil |
| ALCOHOLS | -OH | methanol, ethanol, propanol, butanol, pentanol | Reaction of alkene with water |
| CARBOXYLIC ACID |  | methanoic acid, ethanoic acid, propanoic acid, butanoic acid. | Oxidation of alcohols |
| ESTERS | 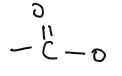 | methyl ethanoate, ethyl ethanoate | Reaction of alcohols and carboxylic acid |
DISCLAIMER
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons



