This page contains the detailed and easy notes for Edexcel IGCSE Chemistry Covalent Bonding for revision and understanding.
Edexcel IGCSE Paper 1: Complete Revision Summary
Covalent Bonding
COVALENT BONDING
- It is between two non metals
- It involves the sharing of electrons between two non metals.
- More than one electron pair can also be shared resulting in the formation of single double and triple bonds.

properties of Covalent Compounds

Simple Molecule
- Eg – O2, CH4
- They have weak intermolecular forces in them so have a lower melting and a boiling points
- The intermolecular forces increases with increase in size as the surface area between the molecules increases.
- Therefore, polymers which have covalent bonding between them have high melting and boiling point due to increase in chain length.
Giant Covalent
- Diamond
- Graphite
- Silicon Dioxide
Ionic Bonding (1)
Edexcel IGCSE Chemistry Covalent Bonding
FREE DOWNLOAD
Send download link to:
Edexcel IGCSE Chemistry Covalent Bonding
Send download link to:
GIANT COVALENT STRUCTURES
Substances which have huge network of atoms joined together by covalent bonds form giant covalent structures.


| DIAMOND | GRAPHITE |
| It is hard. | It is soft and greasy. |
| It is an insulator | It is a conductor |
| It has a high density. | It has a lower density than diamond. |
| Each carbon atom is covalently bonded to four other carbon atoms giving it a strong rigid structure | Carbon atoms are bonded in the form of layer in the form of hexagons. No covalent bonding between the layers so they can slide past. Each carbon atom is bonded with three other carbon leaving the fourth electron has delocalized |
| No delocalised electrons present | It has delocalised electrons |
| Used in cutting or jewellery | It is used in pencil leads. |
PROPERTIES OF GRAPHITE
Q1 Why graphite is soft and slippery?
In graphite, Carbon atoms are bonded in the form of layers in the form of hexagons. No covalent bonding between the layers so they can slide past each other. The layers have only weak intermolecular forces between them. By applying a little pressure then layers can easily slide past each other making Graphite soft and slippery.

Q2 Why graphite conduct electricity ?
In graphite, Carbon atoms are bonded in the form of layer in the form of hexagons. No covalent bonding between the layers so they can slide past. Each carbon atom is bonded with three other carbon leaving the fourth electron has delocalized. These delocalized electrons are mobiles electrons which can move and conduct electricity.
FULLERENE AND GRAPHENE
Fullerene: Hollow shaped molecule having hexagonal rings like a bucky ball.

- Also known as bucky ball or buckminsterfullerene.
- Carbon can be in the form of pentagon or hexagon rings
- Used as catalyst, drug delivery and treating cancer.
- Graphene: Layer of interlocking hexagonal rings like single sheet of graphite.

- It is a better conductor than graphite, light and have low density.
- Used in making computer chips and flexible electronic displays.
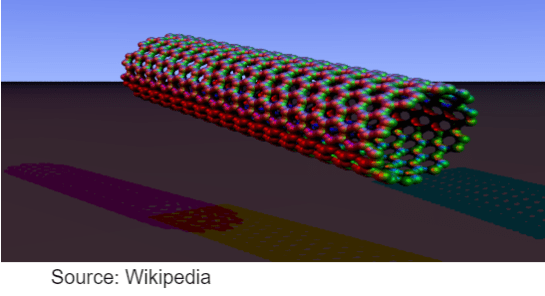
CARBON NANOTUBES
- Cylinderical fullerene with the length greater than the diameter.
- High tensile strength – Used in making reinforced composite materials
- High electronic conductivity – used in electronic industry
DISCLAIMER
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it. References: BBC Bitesize Wikipedia Wikimedia Commons

