Home Edexcel IGCSE Chemistry Carboxylic Acid
Welcome!Log into your account
Weak Acids
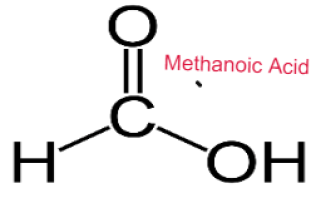
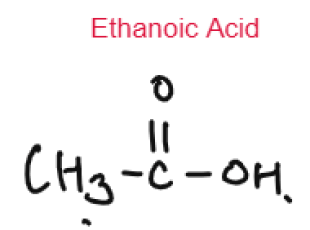
Carboxylic Acids are weak acids as they are partially dissociated in water to release H+ ions.
CH3COOH ⇌ CH3COO– + H+
Metal oxides and Metal hydroxide
Carboxylic Acid reacts with metal oxides and metal hydroxide to form salt and water.
CH3COOH + NaOH CH3COONa + H2O
Metal carbonate
Carboxylic Acid reacts with metal carbonate to form salt, water and carbon dioxide.
CH3COOH + Li2CO3 CH3COOLi + CO2 + H2O

Send download link to:
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
As the title says, this group is for anyone who is currently in GCSE and A-levels and want expert advice on preparing for Maths and Science. All Exam Boards students can join. Here you can find support from Experts and your peers on any issues you are facing related to exam and studying for GCSE and A-levels Maths and Science. The passed outs who wish to help the current students can also join to share their advice. We’re here to share valuable content, exchange ideas, ask each other for help, and share/connect with other members.
You can Join Or Group here –

[sibwp_form id=2]