ALCOHOLS – Have functional Group –OH
General Formulae
CnH2n+1 OH
Formed by replacing hydrogen of alkane with OH group
Used as fuel, solvents, spirits


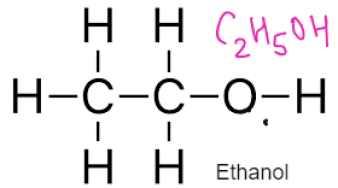

Alcohol
Edexcel IGCSE Chemistry Alcohol
FREE DOWNLOAD
Send download link to:
Edexcel IGCSE Chemistry Alcohol
Send download link to:
REACTIONS OF ALCOHOLS
COMBUSTION
- It can undergo complete or incomplete combustion. Complete combustion produces carbon dioxide and water.
- Ethanol + Oxygen = Carbon dioxide + water
C2H5OH + O2 CO2 + H2O
- Incomplete combustion produces carbon dioxide and water.
- Ethanol + Oxygen = Carbon monoxide + water
C2H5OH + O2 CO + H2O
OXIDATION
- Alcohols are oxidised to carboxylic acid in the presence of oxidising agent.
- Methanol Methanoic Acid
- Ethanol Ethanoic Acid
- Oxidising agent used is acidified potassium dichromate solution
METAL
Alcohols react with metals to form salt and hydrogen.
2C2H5OH + 2Ca 2C2H5OCa + H2
DISCLAIMER
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons

