Mass Spectrometry
Mass Spectrometry is a process by which the atomic mass of atoms or molecules is determined. It can be used to find relative isotopic abundance, atomic and molecular mass, and the structure of a compound.
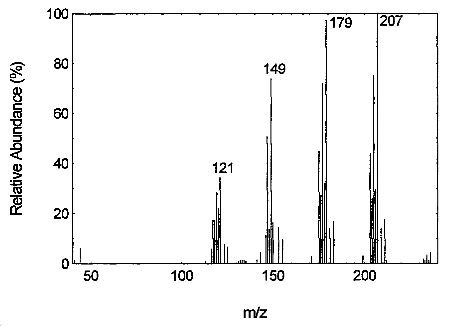
The result of a Mass Spectrometry is a graph plotting mass per charge against relative abundance. Objects (atoms or groups of atoms) of different masses may be detected due to varying atomic masses giving Isotopes and the fragmentation of molecules into smaller groups of atoms. This data can then be used to calculate relative isotopic abundance, atomic or molecular mass, or the structure of a compound.
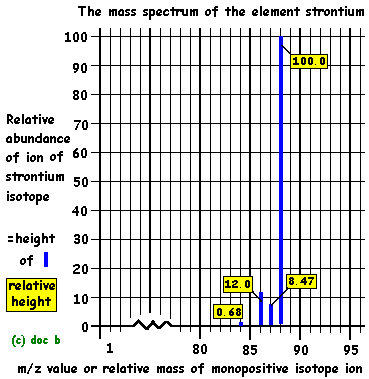
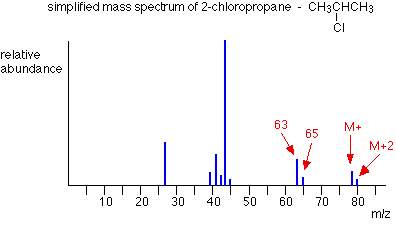
- The peak with the highest mass is called the Molecular Ion, and the peak with the greatest abundance (largest size) is called the Base Peak.
Time-of-Flight Mass Spectrometer
- A Time-of-Flight Mass Spectrometer works by accelerating an ionised sample and calculating mass per charge based on how long each ‘object’ is in flight for. Since every ‘object’ receives equal force, according to Newton’s Second Law, the acceleration of each ‘object’ will be inversely proportional to its mass.
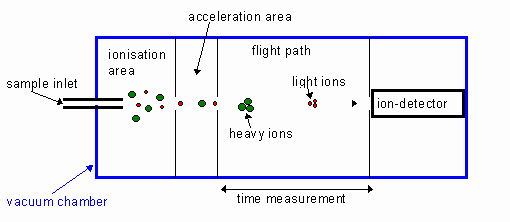
The sample is first ionised by bombarding it with electrons, which also causes fragmentation to form smaller groups of atoms. Ions tend to have +1 charge, since a bombarding electron will knock an electron out of an atom’s shell, so ‘mass per charge’ can generally be taken as simply ‘mass’.
The ions are then accelerated by Electromagnetic Field and travel through a vacuum area called the Drift Region, before being detected by the Ion Detector.
Calculations and Deductions
Given a sample of a single element, the relative atomic mass can be calculated by looking at the peaks and performing a simple mean calculation.
The relative molecular mass can be deduced by looking for the Molecular Ion peak, since this will be the peak caused by the whole molecule.
The structure of a molecule can be deduced by looking at the smaller peaks and inferring the structure of those, given the likely combinations of atoms present that could produce that mass. This is because these peaks will be cause by fragments of the whole molecule. The stronger the bond between atoms, the less likely it is to break, and so the lower the abundance of the fragments that would be formed by the breaking of that bond.
In reality, peaks will not be perfectly clear because of the varying mass of individual atoms. However, smaller peaks like this can help to determine the structure of the larger ones.