Bonding
- Compounds are formed when two or more atoms join together, forming Bonds. There are different types of bonds that occur between atoms which give rise to different properties.
Ionic Bonds
- Ionic Bonds form when electrons are transferred from one atom to another, forming charged Ions which are attracted to each other by Electrostatic Forces. Elements tend to loose or gain electrons, forming Ions, to get a ‘full other shell’.
- Ionically bonded substances, such as Sodium Chloride, can from crystals known as Giant Ionic Lattices with bonds forming a network of connections between atoms.
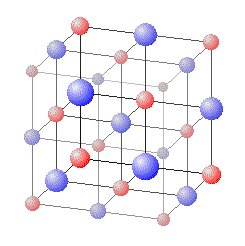
Giant Ionic Lattices have high melting and boiling points since atoms are held together by strong forces.
Ionic substances can conduct electricity through the movement of charged Ions. However, they may only do so if the Ions are free to move around. Therefore Ionic substances conduct electricity when molten or dissolved, but not when in a solid state.
Many Ionic Compounds dissolve in water. This is because the polar water molecules cluster around Ions, and so separate them from each other.
Covalent Bonds
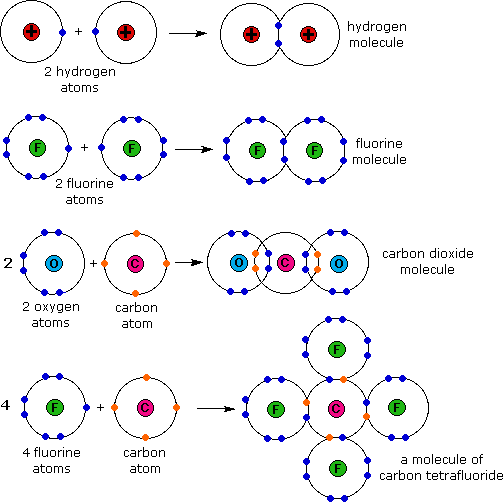
- Covalent Bonds involve the sharing of electrons so that all atoms have ‘full outer shells’.
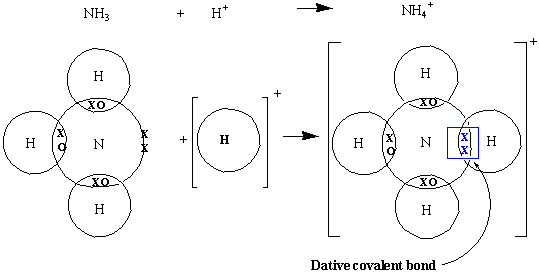
- Sometimes in a Covalent Bond, both shared electrons come from the same atom. This is known as a Dative Covalent Bond. This often results in the formation of charged molecules.
Simple Molecular Structures
Substances composed of relatively small covalently bonded structures are called Simple Molecular Structures.
Simple Molecular Structures tend to have low melting and boiling points since the forces between molecules (intermolecular forces, which are van der Waals forces) are quite weak.
They don’t tend to conduct electricity, since there are no free charge carriers.
They tend to be quite insoluble in water, but this depends on how polarised the molecule is. The more polar the molecules, the more water molecules will be attracted to them.
Giant Covalent Structures
- Sometimes, as is the case with Carbon, covalently bonded structures can form giant networks, known as Giant Covalent Structures. In these structures, each a network of bonds connects all the atoms to each other.
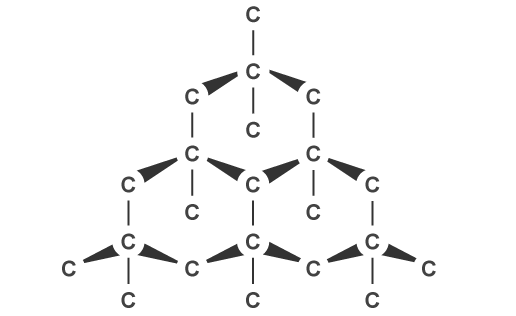
These structures are usually very hard and have high melting and boiling points. This is because of the strong covalent bonds holding each atom in place.
In general, Giant Covalent Structures cannot conduct electricity due to the fact that there are no free charge carriers. One notable exception to this is Graphite. This is a structure composed of ‘sheets’ of carbon atoms on top of each other. Electrons can move between the sheets and so carry electricity.
Metallic Bonding
- Metals form Giant Metallic Lattices. These are composed of positive metal ions surrounded by a ‘sea’ of Delocalised Electrons. The metal ions are attracted to the negative electrons.
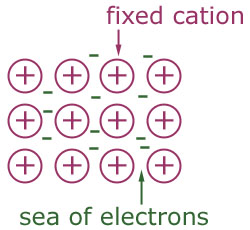
Metals tend to have high melting and boiling points because of the attraction between the metal ions and the electrons. The more Delocalised Electrons are present (because of a higher valency), the greater the melting and boiling points.
Metals conduct electricity because the electrons are free to move and carry charge.
Metals do not tend to dissolve, except in liquid metals, due to the strength of the attraction between the metal ions and the electrons.
Metals are Malleable and Ductile. This is because there are no direct bonds between metal ions, so they can slide over each other.
The Shapes of Molecules
Different molecules have different shapes. The shape of a molecule is dictated by the arrangement of Electron Pairs. This is because Electron Pairs repel each other. The molecule settles in a state where the Electron Pairs are furthest apart from each other.
- Different types of Electron Pairs have different repulsions. There occur Lone Pair Electrons (LP) and Bonding Pair Electrons (BP). The order of repulsion between different combinations, in order of decreasing repulsion, and so decreasing angle, is:
- LP - LP
- LP - BP
- BP - BP
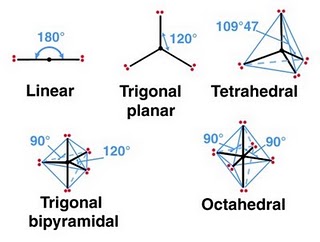
- Common bond angles:
- 2 Electron Pairs on a single atom form 180° bond angles.
- 3 Electron Pairs on a single atom form 120° bond angles.
- 4 Electron Pairs on a single atom form 109.47(1220… =
)° bond angles.
- 6 Electron Pairs on a single atom form 90° bond angles.
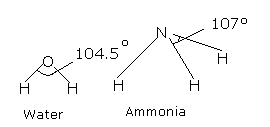
- 2 Lone Pairs and 2 Bonding Pairs on a single atom form a 104.5° bond angle.
- 1 Lone Pair and 3 Bonding Pairs on a single atom form a 107° bond angle.