Osmosis
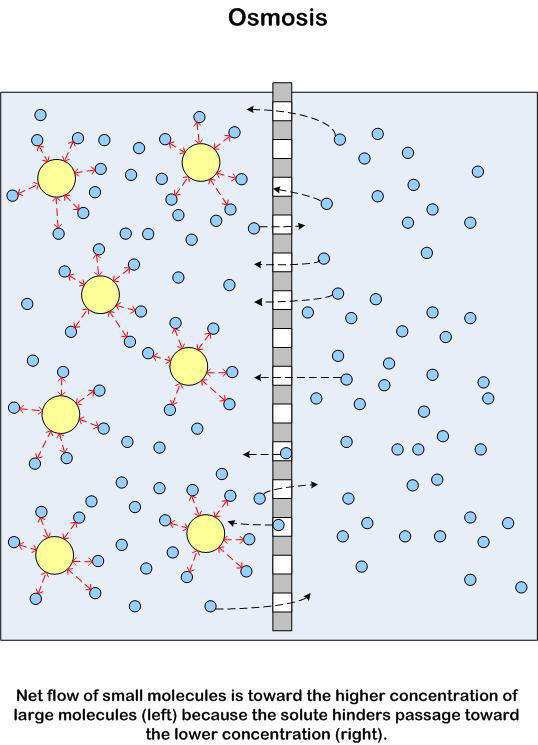
Osmosis is the diffusion of water through a Partially Permeable Membrane. It is a special case of Diffusion in that the concentrations of Solutes in the water can effect how it occurs.
Since water is a Polar molecule, many substances dissolve in it. These dissolved substances are termed Solutes, and water is a Solvent. Water molecules cluster around molecules of a Solute.
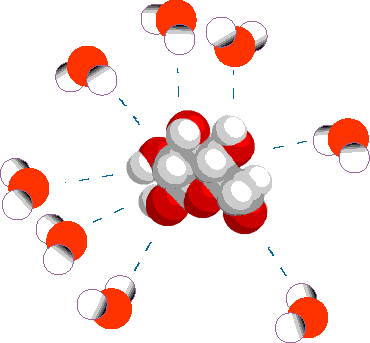
Because some water molecules cluster around a Solute when it is dissolved, there are less ‘free’ molecules which can diffuse to other areas. This effectively lowers the concentration of water.
Water Potential measures the concentration of free water molecules. It is a measure of the tendency of these molecules to diffuse to another area. The more free water molecules, the higher the Water Potential.
Water diffuses by Osmosis from a region of high Water Potential to a region of low Water Potential through the Water Potential Gradient. Osmosis can therefore be defined as the diffusion of water from a region of high Water Potential to a region of low Water Potential through a Partially Permeable Membrane.
- Water Potential is measured in kiloPascals (kPa), where the Highest Water Potential (that of pure water) is 0 kPa and lower Water Potentials go into negative numbers.
Osmosis and Cells
Since cells contain various Biological Molecules, such as Sugars and Salts, they have a Water Potential lower then 0 kPa. Water may move in or out of a cell depending of the Water Potential Gradient between the inside of the cell and its environment.
When water diffuses into a plant cell, when it is placed in a solution of higher Water Potential than inside it, the cell contents will expand. However, since plant cells are surrounded by a strong cell wall, they will not burst. The cell contents will push against the cell wall, and the cell will become Turgid.
If a plant cell is placed in a solution of lower Water Potential, water will diffuse out. This causes the Cytoplasm to shrink and become Flaccid. If enough water leaves, the Cytoplasm will pull away from the cell wall. The cell will become Plasmolysed.
Animal cells will also expand when they are placed in a solution of higher Water Potential. Since animal cells do not have cell walls, if this happens excessively the cell will burst open and become Haemolysed.
If water leaves an animal cell by Osmosis, it will shrink and appear ‘wrinkled’. It will become Crenated.